Neha Rajpal, BA 1; Elliot T. Walters, MD 2; Tammer Elmarsafi, DPM 2; Troy A. Pittman, MD 3; Kelly K. Johnson-Arbor, MD 2
CORRESPONDING AUTHOR: Kelly K. Johnson-Arbor – kkja@me.com
Introduction: Mastectomy skin flap necrosis represents a significant complication of breast reconstructive procedures and is reported to occur in 30%-52% of patients undergoing breast reconstruction. Early identification of ischemia and early initiation of hyperbaric oxygen (HBO2) therapy can mitigate the effects of ischemia and rescue otherwise non-viable breast flap tissue.
Methods: We retrospectively examined the outcomes of HBO2 therapy in eight breasts with compromised mastectomy skin flaps between September 2015 and January 2017. Indocyanine green angiography (ICGA) was used to assess perfusion intraoperatively and post-HBO2 administration.
Results: Seven patients were referred for HBO2 within 24 hours of mastectomy. One patient failed to improve despite starting hyperbaric treatment within 24 hours. All other patients manifested successful healing of their mastectomy skin flaps with acceptable cosmesis after 10 HBO2 treatments. The mean relative perfusion of the at-risk area was 13.8% (±3.7%) pre-HBO2 and 101.6% (±37.3%) post-HBO2. The average area at-risk pre-HBO2 was 17.1 cm2 and reduced to zero post-HBO2. Relative perfusion values after HBO2 were found to be 6.8 (±3.4) times greater than those measured prior to HBO2.
Conclusions: A short course of HBO2 may be sufficient to successfully rescue at-risk post-mastectomy breast flaps. ICGA is a useful adjunct for evaluating post-mastectomy breast flap perfusion before and after HBO2 therapy.
A significant complication of breast reconstructive procedures, mastectomy skin flap necrosis may occur in 30%-52% of patients undergoing breast reconstruction [1,2]. Early implementation of hyperbaric oxygen (HBO2) therapy may be particularly valuable among patients at risk for mastectomy skin necrosis. HBO2 can enhance neovascularization and flap perfusion through improved oxygen tension, collagen synthesis, and fibroblast function [3].
Evidence suggests that clinical judgment alone is a poor predictor of post-surgical complications [4,5]. Recently surgeons have expanded the application of indocyanine green angiography (ICGA) to plastic reconstructive procedures to determine flap viability [6,7]. ICGA has accurately detected nipple hypoperfusion intra-operatively and proved more accurate in predicting ischemia when compared to clinical judgment alone [4,5,8-21]. Intraoperative use of ICGA may also result in decreased postoperative complications [12].
When ICGA suggests flap hypoperfusion, HBO2 may be administered to prevent progression to flap necrosis. While the benefits of HBO2 for the treatment of breast flap compromise and necrosis are well established, the number, duration, and frequency of treatments required have not been well described. The primary aim of this paper is to present a regimen for the use of HBO2 in patients with breast skin flap compromise and to discuss the utility of ICGA in evaluating the efficacy of HBO2 in the treatment of compromised skin flaps.
This was a single-center, retrospective, IRB-approved case series of eight female breast cancer patients who received nipple-sparing mastectomy (NSM) with reconstruction and HBO2 between September 2015 – January 2017 at MedStar Georgetown University Hospital.
UHM 2019, VOL. 46 NO. 4 – HBO2 FOR ISCHEMIA AFTER BREAST RECONSTRUCTION
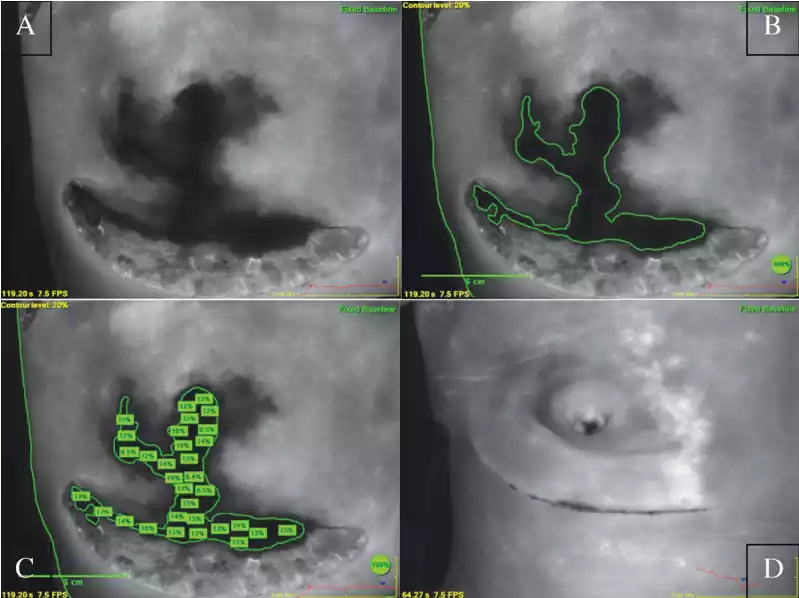
FIGURE 1
(Post-mastectomy ICGA images)
All eight patients underwent NSM. One patient was identified as having severely compromised flaps prior to reconstruction; consequently, immediate reconstruction was deferred in this patient. The remaining seven patients received immediate breast reconstruction using tissue expanders or implants. Tissue expanders were not inflated with saline until after the completion of HBO2 therapy. Breast closure was performed without tension.
Intraoperative skin flap viability was evaluated by a single reconstructive surgeon using ICGA immediately following mastectomy and reconstruction. For the ICGA procedure, a 5cc bolus of indocyanine green dye was administered through a peripheral intravenous line followed by a 10cc saline flush. A SPY camera (Novadaq Technologies, Inc., Toronto, Canada) was positioned appropriately over the breast so as to include the xiphoid process, and a 120-second video of the infusion was obtained. Still, images were captured at approximately 100 seconds. Perfusion was assessed by comparing the fluorescence of the darkest area of interest to the fluorescence at a control area located over the xiphoid process. The decision to treat the patient with HBO2 was made at the end of each surgery based on the post-closure ICGA fluorescence. In line with the standard of care at our institution, ICGA fluorescence values of ≤20% of the control area indicate tissue at risk for postoperative ischemia (Figure 1a) and prompt a referral for HBO2 treatment. ICGA images were assessed retrospectively using the SPY-Q Analysis Toolkit (Novadaq Technologies, Inc., Toronto) to determine relative perfusion (a percentage of fluorescence compared with the control area) of the at-risk region for pre and post-HBO2 images. ImageJ software (NIH, Bethesda, Maryland) was used to calculate the area (in cm2 ) of the image with <20% relative fluorescence intensity for each breast, also known as the “contour” (Figure 1b) [22].
HBO2 was performed using a monoplane hyperbaric chamber (Perry Baromedical Corp., Riviera Beach, Florida, U.S.) pressurized to a depth of either 2.0 or 2.5 atmospheres absolute (ATA). The treatment depth for each patient was determined by a hyperbaric medicine physician according to the patient’s underlying medical comorbidities. Treatments were administered twice daily on weekdays, and once daily on weekends, for a minimum of 10 treatments per patient. Flap perfusion was assessed using ICGA immediately following the final hyperbaric treatment.
SAS 9.4 statistical software (SAS Institute, Cary, North Carolina, U.S. ) was used for all statistical analysis.
Demographics and comorbidities of the eight patients included in this study are listed in Table 1. Seven patients received immediate breast reconstruction with tissue expanders or implants prior to referral for HBO2. Breast reconstruction was delayed in one patient due to extensive intraoperative ischemia with resultant necrosis and infection. Seven patients were referred for HBO2 and began treatment within 24 hours of mastectomy. HBO2 was administered at a depth of 2.5 ATA in seven patients, and at 2.0 ATA in one patient due to a history of seizures. There were no adverse events related to HBO2 reported in this patient population.
One patient failed to improve despite starting hyperbaric treatment within 24 hours. Her pre-HBO2 perfusion percentage was 11.7% and increased to 17.1% after completing 17 HBO2 treatments. This patient failed to improve adequately and was returned to the operating room for debridement of the nipple-areola complex. All other patients manifested successful healing of their mastectomy skin flaps with acceptable cosmesis after 10 HBO2 treatments.
Two patients did not complete the post-treatment ICGA and were excluded from the ICGA analysis. However, after completing HBO2 treatment these patients’ breast flaps recovered without requiring additional intervention. The remaining patients mean relative values of perfusion pre- and post-HBO2 are illustrated in Table 2.
Early initiation of HBO2 following mastectomy has been shown to improve breast flap survival, thereby reducing the risk of infection, esthetic deformity, and implant extrusion [23-25]. The results of this case series demonstrated a greater than sixfold increase in relative perfusion values between pre-and post-HBO2, indicating the potential of HBO2 to improve flap viability and avoid skin necrosis.
Although HBO2 is a recognized treatment for compromised skin grafts and flaps, the available evidence for HBO2 therapy to treat mastectomy flap hypoperfusion is limited. Our results suggest that flap salvage can be accomplished with a limited number of HBO2 treatments, especially when patients are referred for HBO2 within 24 hours of mastectomy and reconstruction.
This case series demonstrates that ICGA represents a valuable adjunct for monitoring response in patients receiving HBO2 therapy. Limitations of this case series include a small sample size and the retrospective nature of the study. In this study, patients were referred for HBO2 within 24 hours of mastectomy; as the referral patterns for HBO2 are variable, the results of this study may not be generalizable to all institutions. In addition, ICGA poses additional limitations, as the timing of injection and image capture are operator-dependent. Future studies should explore the utility of ICGA for monitoring the response of HBO2 therapy over the course of the treatment, as this may help identify the point of maximal effect during the HBO2 treatment course.
TABLE 1. Patient demographics | ||
Patient Demographics | n=8 | range |
age | 49.6 (±9.3) | 40-65 |
Race White African American other |
4 (50%) 3 (37.5%) 1 (12.5%) | |
BMI | 25.6 (±9.7) | |
Comorbidities Hypertension Hyperlipidemia smoking |
2 (25%) 3 (38%) 1 (12%) | |
Table 2. Relative perfusion percentages pre- and post-HBO2 within the at-risk region for patients with successful HBO2 treatment | |||
Patient | Pre-HBO2 | Post-HBO2 | P-value |
1 | 16.3±3.7 | 138.7±36.1 | <.0001 |
2 | 13.3±2.3 | 117.8±35.0 | <.0001 |
3 | 15.1±4.2 | 76.9±28.7 | <.0001 |
4, right | 12.0±3.5 | 114.3±30.5 | <.0001 |
4, left | 13.7±4.1 | 94.0±32.7 | <.0001 |
5 | 13.8±2.9 | 65.3±27.6 | 0.0006 |
6 | 14.8±1.7 | 78.2±22.1 | <.0001 |
mean | 13.8±3.7 | 101.6±37.3 | <.0001 |
In this series of patients with intraoperative identification of mastectomy flap compromise, tissue expander and implant reconstructions were successfully salvaged with a short course of HBO2. ICGA obtained in six patients demonstrated a greater than sixfold increase in relative perfusion values between pre-and post-HBO2, indicating the remarkable capacity of HBO2 therapy to improve flap survival and prevent progression to necrosis. In patients with compromised skin flaps after mastectomy, ICGA can be used to guide and monitor a patient’s response to HBO2 therapy.
Acknowledgments
Novadaq Technologies, Inc., provided the SPY camera and ICGA dye kits used in this study.
Copyright of Undersea & Hyperbaric Medicine is the property of Undersea & Hyperbaric Medical Society and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.
We have locations in Arlington, Flower Mound, Keller, Lewisville, Stone Oak, Kingwood, Frisco, Pearland Texas. Contact one of our offices to make an appointment to begin healing today.
WOUND CARE AND HYPERBARIC OXYGEN THERAPY
Located in The Dallas-Fort WORTH, Houston and San Antonio Areas of Texas