Nicole E Spruijt1 , Roy van den Berg1
1 Da Vinci Clinic, Nieuwendijk 49, 5664HB Geldrop, the Netherlands
Corresponding author: Dr Nicole E Spruijt, Da Vinci Clinic, Nieuwendijk 49, 5664HB Geldrop, the Netherlands n.spruijt@davincikliniek.com
Radiotherapy; Soft-tissue radionecrosis; Hyperbaric medicine; Pain
(Spruijt NE, van den Berg R. The effect of hyperbaric oxygen therapy treatment on late radiation tissue injury after breast cancer: A case-series of 67 patients. Diving and Hyperbaric Medicine. 2020 September 30;50(3):206–213. DOI: 10.28920/ dhm50.3.206–213. PMID: 32957121.) Introduction: Late radiation tissue injury (LRTI) after breast cancer may benefit from hyperbaric oxygen treatment (HBOT). This study aimed to report the LRTI symptom scores up to 12 months after HBOT and identify risk factors for poor scores. Methods: A case series of 67 patients who underwent a mean of 44 sessions of HBOT was analyzed. LRTI symptoms were scored at four-time points using the LENT-SOMA scale (Late Effects in Normal Tissues – Subjective, Objective, Management, and Analytic), a visual analog scale for pain, and the range of shoulder motion. Results: Between starting HBOT and 12 months after HBOT 57 patients (85%) reported at least one point improvement in their LENT-SOMA score. Median pain and fibrosis scores improved significantly between the start and end of HBOT (P < 0.001), and remained stable three and 12 months after HBOT. The median breast oedema score improved significantly 12 months after HBOT (P = 0.003). Median shoulder abduction increased significantly from 90 to 165 degrees (P = 0.001) and median shoulder anteflexion increased significantly from 115 to 150 degrees (P = 0.004). Various risk factors were identified for poor scores despite HBOT; the most common risk factor was a poor score at the start of HBOT. Conclusions: In this case series, patients who underwent HBOT for LRTI after breast cancer reported significant improvement in pain, fibrosis, oedema, and shoulder movement. The improvement persisted up to 12 months after HBOT. A poor score at the start of HBOT was predictive of a poor score 12 months after HBOT.
Breast cancer is the most common form of cancer affecting women in the Netherlands. One in seven women will get breast cancer during their lives.1 It is often diagnosed at an early stage and has a good prognosis; the five-year survival is 87%.1 Survivor faces several late sequelae of treatment including late radiation tissue injury (LRTI). Radiation injury can be divided into acute and late tissue injury.2 Acute injury occurs during or a few months after radiotherapy and is usually self-limiting, including hematoma, dermatitis, breast pain, and implant infection.3 Late injury that persists at six months or occurs over six months after radiotherapy, and usually worsens with time.4
The most common LRTI symptoms after breast cancer are pain, oedema, fibrosis, and limited range of motion of the arm at the shoulder joint.5 The prevalence of LRTI increases with time.4,6,7 For example, five years after radiotherapy for breast cancer 15–19% of patients have moderate to marked breast fibrosis, and 10 years after radiotherapy the incidence increases to 22–28%.7 Although breast pain is common after breast-conserving surgery and radiotherapy (47% of patients report having pain), over 85% of patients consider it tolerable.8
For those who suffer from LRTI, hyperbaric oxygen treatment (HBOT) has been shown to improve symptoms.9–11 Both the European Committee for Hyperbaric Medicine and the Undersea and Hyperbaric Medical Society accept LRTI as an indication for HBOT.2,12 During HBOT, stem cells within irradiated tissues are induced and mobilized and angiogenesis is stimulated, improving tissue oxygenation and decreasing fibrosis.2 The objective of this study was to report the effects of HBOT on LRTI symptoms after breast cancer up to 12 months after completing HBOT and to identify risk factors for persistent symptoms after HBOT.
Data were collected prospectively, recorded in the patient’s medical records, and analyzed retrospectively. In accordance with the Health Code of 2005 based on the Code of Good Conduct 1995, our institutional review board grants a universal waiver for retrospective chart reviews, such as this study.
Figure 1 Diagram showing the compression, oxygen breathing periods, air breaks, and decompression time of a treatment session. MSW – metres’ seawater equivalent ‘depth’
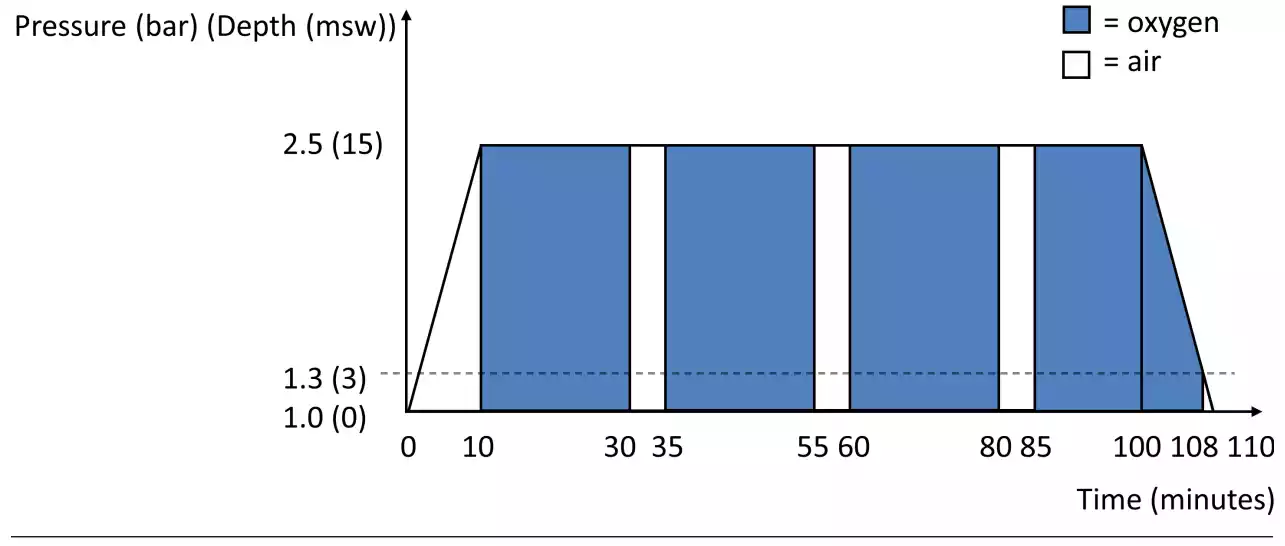
Table 1
Definition of LENT-SOMA scores 5
Symptom | 0 | 1 | 2 | 3 | 4 |
Pain | Absent | Rarely, minimal | Intermittent, tolerable | Permanent, intense | Always, excruciating |
Fibrosis | None | Barely palpable | Definite increased density and firmness | Marked density, retraction, fixation | |
Breast oedema | None | Asymptomatic | Symptomatic | Secondary dysfunction |
All patients who were referred for HBOT for LRTI after breast cancer were candidates for HBOT. Patients underwent an assessment of their fitness for hyperbaric exposure and were prescribed 40 sessions of HBOT. Patients were treated in a multi-place hyperbaric chamber capable of accommodating up to 12 patients (IHC Hytech, Raamsdonkveer, the Netherlands) at an ambient pressure of 253 kPa (2.5 atmospheres absolute). At this pressure, 100% oxygen was breathed via a mask during four periods for a total of 83 minutes, interspersed by three 5-min air breaks (Figure 1). Including compression and decompression time, the total duration of each session was 110 minutes. Patients underwent one session per day, five days per week. Per protocol, 40 sessions were prescribed, but treatment was continued until patients chose to prematurely stop the treatment, the symptoms did not show any further improvement or the maximum number of treatments which are reimbursed by the health insurance was reached (60 sessions). Side effects of HBOT were recorded including problems equalizing the ears, changes in vision, and fatigue.
LRTI symptoms were scored by two dedicated nurses at four-time points: in person before commencing HBOT and upon completion of the course of HBOT, and by telephone three and 12 months after completing HBOT. Three scoring systems were used to quantify LRTI symptoms: 1) the LENT-SOMA (Late Effects in Normal Tissues – Subjective, Objective, Management, and Analytic) scoring system;5 2) a visual analog scale (VAS) to quantify the intensity of pain; and 3) the range of motion in degrees at the shoulder joint on the affected side. Since the range of motion could only be assessed clinically, this was only scored at the start and end of HBOT, and not at the three- and 12-month follow-ups. The LENT-SOMA scoring system is considered the most effective validated tool to analyze the late effects of radiotherapy.13 It is comprised of 12 questions about pain, oedema, fibrosis, telangiectasias, atrophy/retraction, and ulceration, each of which is scored on a 2−5-point scale. In this study, we only report on the primary symptoms for referral to our clinic which was pain, breast oedema, and/or fibrosis (Table 1). The LENT-SOMA scores for pain (0–4), breast oedema (0–3), and fibrosis (0–3) were also summed (score range 0–10).
We used descriptive statistics to describe the patient characteristics, the HBOT course, the side-effects of HBOT, and outcomes after HBOT. We reported the median scores of the LRTI symptoms at each time point. The Kruskal-Wallis
Table 2
Patient characteristics (n = 67)
Characteristic | Mean (range) |
Age (years) | 59 (43−79) |
Body mass index (kg∙m-2) | 27.8 (18.8−43.9) |
n (%) | |
Smoking: Never Stopped Current |
25 (37) 34 (51) 8 (12) |
Breast surgery: Breast-conserving Mastectomy |
50 (75) 17 (25) |
Axillary nodes: Sentinel node removal Axillary clearance Axillary radiotherapy |
36 (54) 25 (37) 6 (9) |
Chemotherapy | 46 (69) |
Time since radiotherapy: < 1 year 1−3 years 3−5 years > 5 years |
26 (39) 20 (30) 2 (3) 19 (28) |
test was used when comparing the median pre- and postHBOT scores of more than two groups with a P-value limit of the significance of < 0.05. If this overall test was significant, the Mann-Whitney U test was then used to compare pairs of groups. The Spearman correlation test was used for the evaluation of correlations between risk factors and the LENT-SOMA and VAS scores 12 months after HBOT. Stepwise multivariate logistic regression analyses were used to assess associations between risk factors and LENT-SOMA pain, fibrosis, or breast oedema score ≥ 2 and VAS score ≥ 5 at 12 months after HBOT. No correction for multiple testing was employed. All analyses were performed using SPSS software (SPSS inc. version 22.0, Chicago, IL).
Between November 2015 and December 2017 a total of 101 patients presented with LRTI after breast cancer; 97 were offered HBOT and 91 accepted treatment. Ten of these patients (11%) prematurely stopped the treatment: three due to recurrent breast cancer; four due to anxiety/ hyperventilation; and three for other reasons. The supervising physician was always consulted when patients prematurely stopped treatment. Of the 81 patients who completed the HBOT, data were missing for 14 patients (17%) and complete for 67 patients (83% follow-up).
All 67 patients were women whose characteristics are listed in Table 2. Fifty patients (75%) had breast-conserving surgery, 36 patients (54%) only had sentinel node clearance, and 46 patients (69%) had chemotherapy. The time interval between radiotherapy and starting HBOT was less than one year for 26 patients (39%) and longer than five years for 19 patients (28%).
The patients underwent a mean of 44 HBOT sessions (range 26–60). During the HBOT five patients (8%) had difficulty equalizing the ears requiring referral to an otorhinolaryngologist for grommets. Upon completion of the course of HBOT, 46 patients (69%) reported increased fatigue. Transient vision changes were reported in 56 patients (84%). Four patients (6%) had vision changes that persisted three months after completion of the HBOT and were referred to an ophthalmologist: two were found to have cataracts and two had refraction changes, probably not related to the HBOT, requiring new glasses.
IMPROVEMENT WITH HBOT
The median LENT-SOMA pain, fibrosis, their sum, and VAS scores improved significantly between the start and end of HBOT (P < 0.001), and remained stable at three and 12 months after HBOT (Figure 2). Compared to the score at the start of HBOT, the median LENT-SOMA breast oedema score was not significantly lower at the end of HBOT (P = 0.188) nor after three months (P = 0.066), but was significantly lower 12 months after HBOT (P = 0.003).
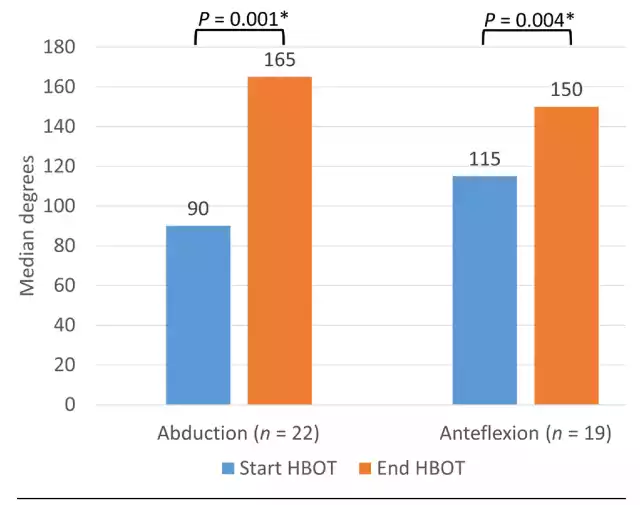
Among the patients who had a limited range of motion of the arm at the start of HBOT, median shoulder abduction increased significantly from 90 to 165 degrees (n = 22, P = 0.001) and median shoulder anteflexion increased significantly from 115 to 150 degrees (n = 19, P = 0.004) at the end of HBOT (Figure 3).
Between the start and end of HBOT, 54 patients (81%) reported at least one point improvement in their LENTSOMA summed score. Between the end of HBOT and three months after HBOT, 29 patients (43%) reported at least one point improvement in their LENT-SOMA sum score. Between three and 12 months after HBOT 23 patients (34%) reported at least one point improvement in their LENTSOMA sum score. Overall, between the start of HBOT and 12 months after HBOT, 57 patients (85%) reported at least one point improvement in their LENT-SOMA sum score, 44 (66%) reported at least one point improvement in their LENT-SOMA pain score, 50 (75%) reported at least one point improvement in their LENT-SOMA fibrosis score and 29 patients (43%) reported at least one point improvement in their LENT-SOMA breast oedema score.
UNIVARIATE CORRELATIONS
Associations between risk factors and LENT-SOMA and VAS scores 12 months after HBOT were analysed by Spearman correlations (Table 3). The LENT-SOMA pain score 12 months after HBOT was correlated with the axillary
Figure 2 Median LRTI symptom scores at the four-time points (n = 67)
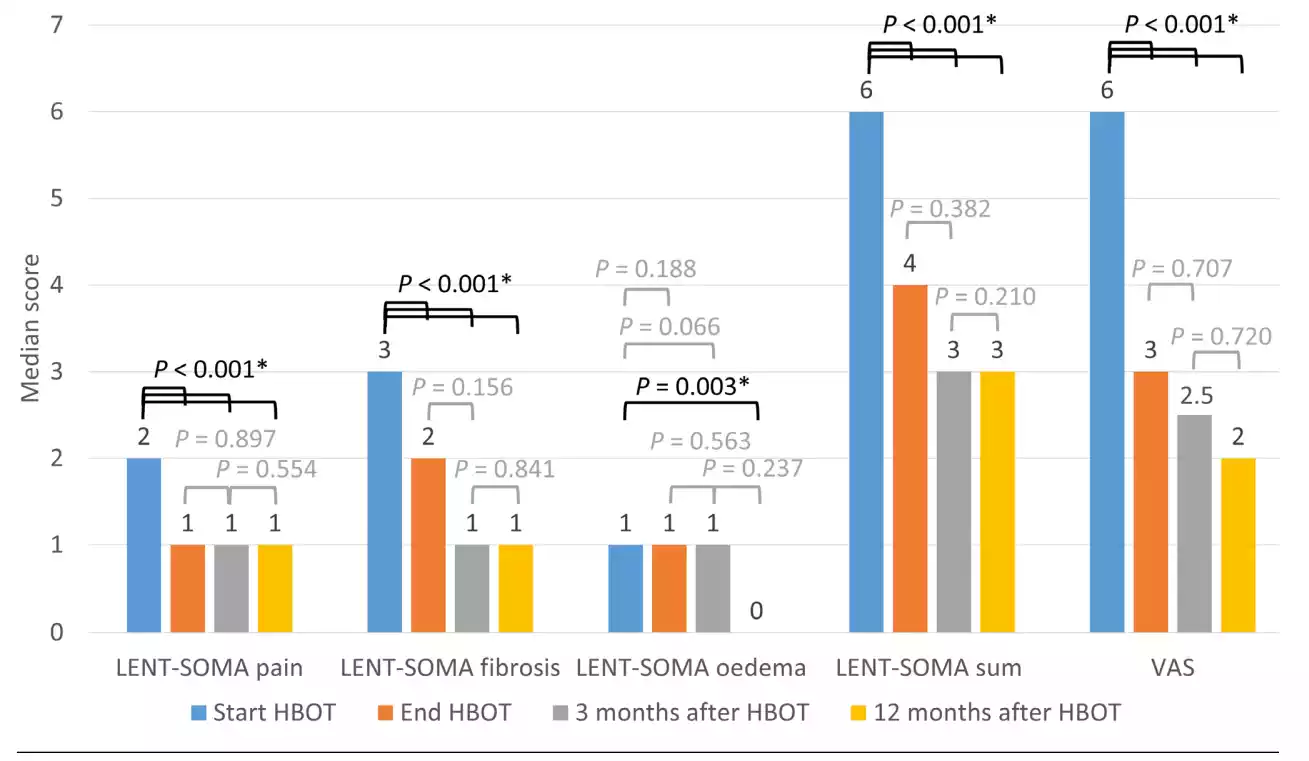
Figure 3 Range of motion from the shoulder joint on the affected side at the start and end of HBOT
node treatment (rho = -0.244, P = 0.046), sessions of HBOT (rho = 0.261, P = 0.033), and the LENT-SOMA pain score at the start of HBOT (rho = 0.684, P < 0.001). The LENTSOMA fibrosis score 12 months after HBOT was correlated with chemotherapy (rho = -0.246, P = 0.045) and the number of sessions of HBOT (rho = 0.243, P = 0.048). The LENTSOMA breast oedema score 12 months after HBOT was correlated with age (rho = -0.302, P = 0.013), body mass index (rho = 0.249, P = 0.043), time since radiotherapy (rho = -0.431, P < 0.001), and the LENT-SOMA breast oedema score at the start of HBOT (rho = 0.647, P < 0.001). The VAS score 12 months after HBOT was correlated with the axillary node treatment (rho = -0.248, P = 0.043), the number of sessions of HBOT (rho = 0.357, P = 0.003), and the VAS score at the start of HBOT (rho = 0.476, P < 0.001).
MULTIVARIATE ANALYSES
Step-wise multivariate logistic regression analyses showed significant associations between specific risk factors and LENT-SOMA pain, fibrosis, or breast oedema score ≥ 2 and VAS score ≥ 5 at 12 months after HBOT (Table 4).
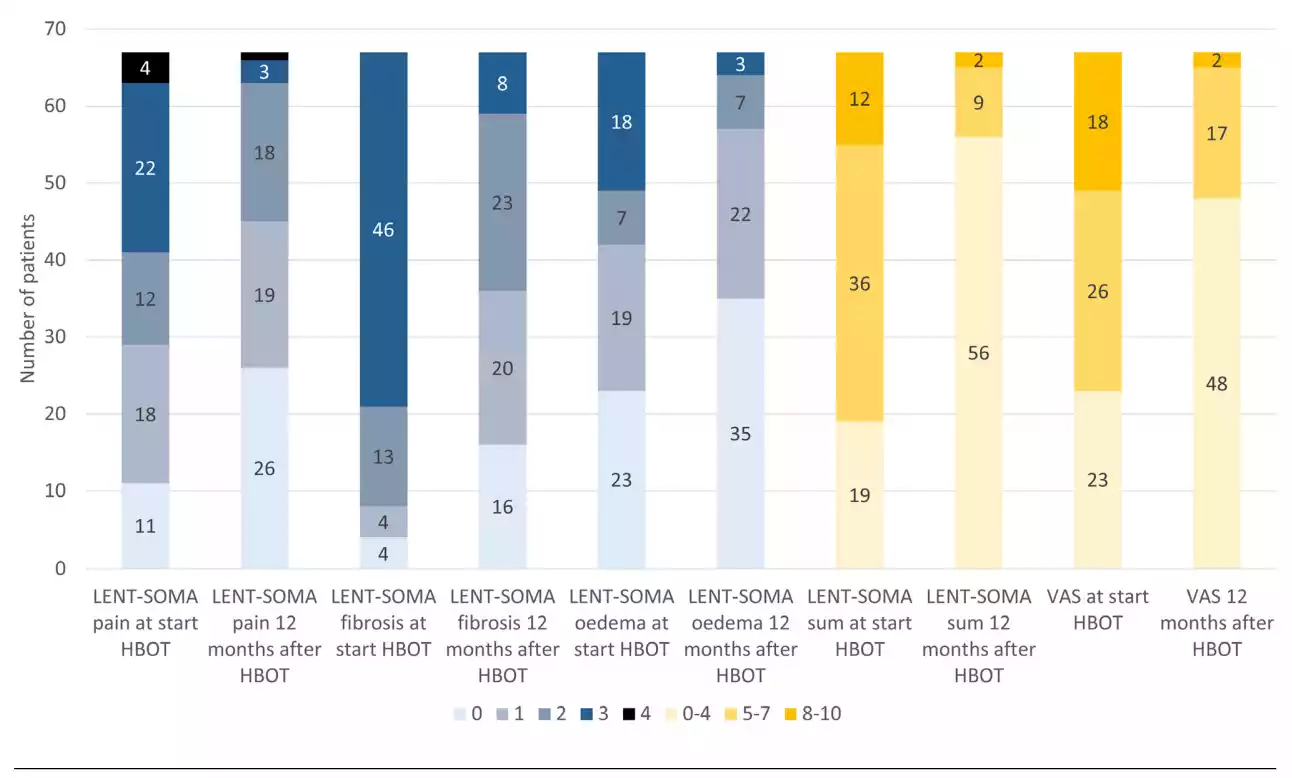
The prevalence of a LENT-SOMA pain score ≥ 2 decreased from 38 patients (57%) at the start of HBOT to 22 patients (33%) 12 months after HBOT (Figure 4). In the multivariate analyses, only a LENT-SOMA pain score ≥ 2 at the start of HBOT was a risk factor for LENT-SOMA pain ≥ 2 at 12 months after HBOT (OR 15.0, 95% CI 3.1–72.2).
The prevalence of a LENT-SOMA fibrosis score ≥ 2 decreased from 59 patients (88%) at the start of HBOT to 31 patients (46%) 12 months after HBOT (Figure 4). In the multivariate analyses, only current smoking at the start of HBOT was a risk factor for LENT-SOMA fibrosis ≥ 2 at 12 months after HBOT (OR 10.2, 95% CI 1.2–88.4).
The prevalence of a LENT-SOMA breast oedema score ≥ 2
Table 3 Correlation between patient characteristics and LENT-SOMA and VAS scores in 67 women 12 months after HBOT; * = statistically significant; # = LENT-SOMA pain, fibrosis, breast oedema, and VAS scores
Characteristic | LENTS O M A pain | P-value | LENTS O M A fibrosis | P-value | LENTS O M A oedema | P-value | VAS | P-value |
Age | -0.162 | 0.191 | -0.208 | 0.092 | -0.302 | 0.013* | -0.156 | 0.207 |
Body mass index | -0.090 | 0.471 | 0.053 | 0.672 | 0.249 | 0.043* | -0.004 | 0.976 |
Smoking | 0.073 | 0.558 | 0.019 | 0.877 | 0.004 | 0.971 | 0.012 | 0.920 |
Breast surgery | -0.234 | 0.057 | -0.100 | 0.421 | -0.057 | 0.648 | -0.129 | 0.297 |
Axillary nodes | -0.244 | 0.046* | -0.231 | 0.060 | 0.070 | 0.571 | -0.248 | 0.043* |
Chemotherapy | -0.065 | 0.604 | -0.246 | 0.045* | -0.052 | 0.676 | -0.077 | 0.537 |
Time since radiotherapy | -0.038 | 0.758 | -0.142 | 0.253 | -0.431 | < 0.001* | -0.031 | 0.806 |
Sessions of HBOT | 0.261 | 0.033* | 0.243 | 0.048* | 0.048* | 0.612 | 0.357 | 0.003* |
Respective score# at start HBOT | 0.684 | < 0.001* | -0.063 | 0.612 | 0.647 | < 0.001* | 0.476 | < 0.001* |
Table 4 Odds ratios for patient characteristics and LENT-SOMA and VAS scores in 67 women 12 months after HBOT; * = statistically significant; # = LENT-SOMA pain, fibrosis, breast oedema and VAS scores
Characteristic | LENTS O M A pain | P-value | LENTS O M A fibrosis ≥ 2 | P-value | LENTS O M A oedema ≥ 2 | P-value | VAS ≥ 5 | P-value |
Age | 0.064 | 0.800 | 0.926 | 0.336 | 0.388 | 0.533 | 1.029 | 0.477 |
Body mass index | 1.069 | 0.301 | 0.435 | 0.510 | 1.082 | 0.298 | 0.989 | 0.125 |
Smoking | 0.021 | 0.885 | 10.21 | 0.035* | 0.039 | 0.843 | 1.831 | 0.489 |
Breast surgery | 0.495 | 0.482 | 0.253 | 0.615 | 0.044 | 0.834 | 3.928 | 0.139 |
Axillary nodes | 0.057 | 0.812 | 3.379 | 0.066 | 0.191 | 0.662 | 0.350 | 0.227 |
Chemotherapy | 0.061 | 0.805 | 3.548 | 0.060 | 1.634 | 0.201 | 1.117 | 0.739 |
Time since radiotherapy | 1.131 | 0.288 | 1.230 | 0.267 | 2.686 | 0.101 | 0.102 | 0.025* |
Sessions of HBOT | 0.227 | 0.634 | 2.844 | 0.092 | 0.125 | 0.724 | 1.047 | 0.314 |
Respective score# at start HBOT | 15.00 | 0.001* | 0.039 | 0.844 | 23.06 | 0.004* | 6.295 | 0.027* |
decreased from 25 patients (37%) at the start of HBOT to 10 patients (15%) 12 months after HBOT (Figure 4). In the multivariate analyses, only a LENT-SOMA breast oedema score ≥ 2 at the start of HBOT was a risk factor for LENTSOMA pain ≥ 2 at 12 months after HBOT (OR 23.1, 95% CI 2.7–197.1).
The prevalence of a VAS score ≥ 5 decreased from 43 patients (64%) at the start of HBOT to 19 patients (28%) 12 months after HBOT (Figure 4). A VAS score ≥ 5 at the start of HBOT (OR 6.3, 95% CI 1.2–32.1) and a time interval ≥ 3 years between radiotherapy and the start of HBOT (OR 0.1, 95% CI 0.01–0.8) were risk factors for VAS score ≥ 5 at 12 months after HBOT in the multivariate analyses.
In this study we add to the literature that shows that symptoms of LRTI after breast cancer improve after HBOT.9–11 Pain and fibrosis scores improved between the start and end of HBOT and remained stable up to 12 months after HBOT. Shoulder range of motion improved between the start and end of HBOT. Breast oedema scores did not improve significantly between the start and end of HBOT, but 12 months after HBOT they were significantly lower than at the start of HBOT. Risk factors were elucidated for high scores 12 months after HBOT. As in previous studies, we chose the cut-off point to be LENT-SOMA scores of ≥ 2.6,14–16
Figure 4 Prevalence of LRTI symptom scores at the start of HBOT and 12 months after HBOT in 67 women; color codes refer to LENT-SOMA scores or score intervals as indicated
HBOT is not the first line of treatment for LRTI symptoms after breast cancer. Patients are referred for HBOT when analgesics, oedema therapy, and/or physiotherapy have not led to satisfactory symptom improvement. In general, patients are very pleased with the improvement in LRTI symptoms following HBOT. They do not expect all the symptoms to resolve with HBOT, and are delighted when the quality of life improves because of decreased pain, fibrosis, or oedema and increased range of motion from the shoulder. We noticed that some patients seem to benefit more from HBOT than others and sought predictive factors. Overall, the most common risk factor for higher scores 12 months after HBOT was a higher score at the start of HBOT (Table 3). Higher scores 12 months after HBOT were correlated with more sessions of HBOT, which corresponds to our intent to tailor the treatment duration to the severity of the patient’s symptoms.10
Specifically, we found that higher pain scores (LENT-SOMA and/or VAS) at 12 months after HBOT were correlated with more aggressive axillary node treatment, a higher pain score at the start of HBOT, and more sessions of HBOT (Table 3). Multivariate analyses showed the risk factors for higher scores 12 months after HBOT were higher pain scores at the start of HBOT (OR 15.0 for LENT-SOMA score and OR 6.3 for VAS score) and a shorter interval between radiotherapy and the start of HBOT (OR 0.1). Other studies have shown that the LRTI pain score is associated with the radiotherapy dose8,15,17 larger breast volume,15 shorter time since radiation,8 and hormone therapy.8
We found that higher fibrosis scores at 12 months after HBOT were correlated with chemotherapy and more sessions of HBOT (Table 3). Multivariate analyses showed the only risk factor for higher fibrosis scores at 12 months after HBOT was current smoking at the start of HBOT (OR 10.2). Other studies have shown that the LRTI fibrosis score is associated with chemotherapy,17,18 larger irradiated volume,18 and increased time after radiotherapy.18
We found that higher breast oedema scores at 12 months after HBOT were correlated with lower age, higher body mass index, a shorter time interval between radiotherapy and the start of HBOT, and a higher oedema score at the start of HBOT (Table 3). Multivariate analyses showed the only risk factor for higher breast oedema scores at 12 months after HBOT was a higher oedema score at the start of HBOT (OR 23.1). Other studies have shown that LRTI breast oedema scores were associated with axillary clearance, breast ptosis, and a bra cup size larger than C.17
The prevalence of LRTI symptoms after breast cancer varies per the study, which can be expected given the variety of variables that may influence the outcome.19 A strength of this study is the heterogeneous population of patients who had undergone different surgeries and types of radiotherapy representing the full spectrum of patients who suffer late sequelae of radiotherapy. On the other hand, the heterogeneous population is also a limitation of this study since it does not answer the question of whether a specific patient will benefit from HBOT. Another limitation is that we do not know details about the radiotherapy our patients had, which has been shown to be an important prognostic factor for LRTI symptoms in other studies.6,8,15,17,18 Since the follow-up scores after three and 12 months were collected by telephone, the reported oedema and fibrosis scores could not be verified with a physical examination. Another limitation is that we performed a per-protocol evaluation rather than an intention to treat (10 patients prematurely stopped treatment). Furthermore, we cannot definitively attribute the improvement of symptoms to the HBOT since we did not inventory concomitant treatment such as the use of analgesics, physiotherapy, or compression. Finally, the greatest limitation of this study is the lack of a control group preventing us from excluding a placebo effect. Given the known progression of LRTI symptoms with time,4,6,7 we assume a control group who did not undergo HBOT would report worsening symptoms during a 12-month follow-up period. Therefore, any future study should be in the form of a randomized, controlled trial.
In conclusion, we found significant improvement in pain, fibrosis, oedema, and shoulder movement scores among patients with LRTI after breast cancer who underwent HBOT. Shoulder movement was not followed up after the completion of the HBOT. The improvement in pain, fibrosis and oedema persisted up to 12 months after HBOT. Clinicians should be aware of this treatment option for patients with LRTI after breast cancer.
Arjette Maas and Irene Stark collected the data.
Conflicts of interest and funding: nil
Submitted: 13 December 2019
Accepted after revision: 14 April 2020
Copyright: This article is the copyright of the authors who grant Diving and Hyperbaric Medicine a non-exclusive license to publish the article in electronic and other forms.
We have locations in Arlington, Flower Mound, Keller, Lewisville, Stone Oak, Kingwood, Frisco, Pearland, Argyle Texas. Contact one of our offices to make an appointment to begin healing today.
WOUND CARE AND HYPERBARIC OXYGEN THERAPY
Located in The Dallas-Fort WORTH, Houston and San Antonio Areas of Texas